Interested in tweaking water for coffee with Mg, then read on…
Intro
Of all the things coffee people like to do most, waxing lyrical about water falls into a weird category, everyone talks about it, but hardly anyone does anything about it.
Most of the time slapping a filter between your water source and the brew water is merely a band aid on a sore, it just makes you feel better for doing something.
On the other end of the spectrum are those that buy RO or distilled water, then tweak the water for their coffee and taste. Perhaps the most popular reference to do this is the Barista Hustle post on water, you can read here.
Of course, there are those that try bottled water, but that itself also opens a worm hole of a mess. Many bottled waters are tested for drinking not brewing. Hence results vary.
What we are proposing here is a small tweak to your brewing water, that you can do with very little hassle.
Trying to summarize what and how in water for coffee in a short form is not easy, but here we go, hold on it may be a tough ride. If you are still keen to read more I suggest the book Water for coffee – by Colonna-Dashwood & Hendon (latest edition being released soon)
Measuring: what is in our water?
There have been books, white papers and fisticuffs (for those that take it most seriously) as to what the perfect water is. The issue really is that the perfect water definitions out there rely on four generally accepted measurements.
What are these?
- Total Dissolved Solids (TDS)
- General Hardness (GH)
- Carbonate Hardness (KH)
- pH
TDS
If I had a Bitcoin for every time this term gets mentioned when it comes to water, I would probably own all the Bitcoin ever mined. What solids are these that are dissolved and where? TDS measures the combined total inorganic and organic substances in a liquid in suspended form. So basically, anything that is not H2O. This is about as reliable a way of assessing what is in water, as licking your finger and placing it in the wind, to figure out the speed the wind is blowing.
GH or dGH
While not as popularized as TDS, this term is also spewed out far too easily by “water” people, specifically when it comes to coffee. It is often assumed that GH measures only the ppm (parts per million of) Calcium (Ca) and Magnesium (Mg). This is a major issue when “designing” water for coffee. GH is a measure of the concentration of divalent metal ions – (wikipedia). What other divalent metal ions are measured? Water likes ions and will collect them on the way to your brew, or others are added. This means that GH can also include Na, Cu, Fe, NH, S, Pb, Zn to name a few.
This means that GH is not a reliable way to accurately measure Mg and Ca in tap water.
KH or dKH
Carbonate Hardness or Karbonathärte, measures the presence of Carbonate (CO3) and bicarbonate (HCO3) anions. Expressed in degrees or in ppm, one degree (1 dKH) corresponds to the carbonate and bicarbonate ions found in a solution of approximately 17.848 milligrams of calcium carbonate(CaCO3) per litre of water (17.848 ppm).
What is important for us here is that this is the buffer to be used for stabilizing pH – “Buffering systems are nature’s way of maintaining a consistent pH range” – Water for coffee by Maxwell Colonna-Dashwood & Christopher H. Hendon.
If the pH of the water is too far away from 7 then KH becomes very important to what we need to do to the water to help it make great coffee.
We are not going to go in to this in this post, since this will be part of the next post on CaCO3.

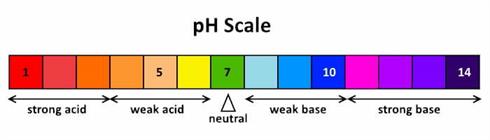
pH
Because coffee wants to be at 5 pH, it is going to draw out of the water what it needs to achieve that. In most cases in a water with a pH north or south of 7, the equipment that heats the water is going to be damaged by the buffer (or any other ions it can find) especially for pH’s below 6 and above 8. If water is low in pH and low in minerals the process of heating and brewing will extract all buffering minerals out of whatever is around, the boiler, the element, or anything it can find since (as stated before) nature wants to maintain a consistent pH range and in coffee that is 5.
Why is this important?
pH or potential of Hydrogen is used to specify the acidity or basicity of an aqueous solution. The ideal pH for brewing coffee is 7. What is of interest here is no matter what the pH of the brew water is, coffee’s pH is always around 5. In our tests we got a pH’s of between 4.9 and 5.1.
What makes good brewing water?
To simplify this, like was done in Water for Coffee (by Colon-Dashwood & Hendon) – there are four important considerations for brewing coffee. The quantity in ppm in the water of Calcium (Ca), Magnesium (Mg) and Bicarbonate (HCO3), and last but not least what is its pH.
You can refer to the graph on the BH post, or as a general rule of thumb, what you want is slightly more Mg to Ca. What suits coffee best is a total ppm of these two of around 100 (depending on who you ask this can be as low as 80 or as high as 120). We believe about 60% of that should be Mg. If the pH of the water is as close to 7 or 7.2 then the level of HCO3 is not that important, if not you need enough to help the water reach a balanced pH during the brew (we will cover HCO3 in the next post).
So how do we translate this knowledge into our brew water? Gently now, read on.
Tweaking water for coffee with Mg
What follows below assumes that the tap water is drinkable (so has a pH between 6 and 8) and is clean enough for a simple water filter to do its job of removing the bulk of the unwanted chemicals (and some we may need). What we will be doing is trying to get the Mg ppm to a level that makes coffee more enjoyable.
Water filtering
The standard off the shelf water filters like the Brita, remove most of the chemicals we do not want in water for our coffee: Cl, Fl, Hg, Cu, Cd and Zn.
Assuming you have some sort of water filtration, or perhaps you want to use spring water, or bottled water that is low in Mg, and has a pH around 7.
So, create at least 500ml of that water to use in the next step.
Creating a Mg Essence
The original formula on creating an Mg Essence is based on the formula in the Water for Coffee book and the interpretation of it on the Barista Hustle water recipe post. The idea here is to create a very diluted form of Mg so that we can add it to our brew water.
Ingredients:
- 5g or Epsom salts
- 500g filtered / spring water
Instructions:
Measure out 5g (about a level teaspoon, but better to measure) of Epsom Salts add to a bottle with filtered water of 500g (use an old 500ml bottle). Shake well.
Making brew water
Now that you have your filtered water and your Mg Essence, you are ready.
We have tried this recipe on Newlands spring water, filtered tap water and a few of the bottled waters with pH around 7.
Method:
- In a 5-litre container with your selected water add 20 g of the Essence to start. (or you can try 5g (a teaspoon) per 1.5 litres of water – a typical kettle / automatic tank)
- Taste the coffee does it taste more “juicy” or complex?
- If you want to try make it a little more interesting add another 5g (1g for 1.5ltr water) of the Essence.
- Repeat adding 5g (1g for 1.5ltr water), until the coffee Is tasting burnt or nasty, you have added enough, go back one 5g (1g).
That is, it.
Just a note: too much Mg can cause an upset stomach (which will return to normal after a visit to Percy), so start small.
By using this recipe, you can ensure there is enough Mg in the water to let the flavoursome compounds in the coffee play nice in the final brew. While this probably gives us the most dramatic effect of all the tweaks you can do, there are further tweaks we will examine in additional posts to come, where we will look at HCO3 and pH.










Great writing and very informed. I like your style!
Thanks for the encouragement.